
T he ability of botulinum toxin to inhibit acetylcholine release at the neuromuscular junction has been exploited for use in medical conditions characterized by muscle hyperactivity. As such, botulinum toxin is widely recommended by international treatment guidelines for movement disorders and it has a plethora of other clinical and cosmetic indications... The chronic nature of these conditions requires repeated injections of botulinum toxin, usually every few months. Multiple injections can lead to secondary treatment failure in some patients that may be associated with the production of neutralizing antibodies directed specifically against the neurotoxin. This is because [conventional formulations of—] botulinum toxin type A [are] a 150 kD protein produced by Clostridium botulinum, which exists in a complex with up to six additional proteins. The complexing proteins may act as adjuvants and stimulate the [undesired; immunotoxicity; hypersensitivity] immune response.”T here have in recent weeks been a variety of inquiries from CMT blog readers about whether there are new developments in the treatment of focal dystonia in musicians. Specifically, the inquiries have concerned two things: (1) the recent FDA approval of a new formulation of botulinum toxin by Merz Pharma, called Xeomin®, and (2) recent clinical trials of an antibiotic, minocycline, that crosses the blood-brain barrier and is known to have certain neuroprotective properties in preclinical in vitro testing and animal models.
— Reiner Benecke, Dept of Neurology, Univ Rostock.
T here is not yet enough evidence regarding minocycline’s efficacy in neurological conditions—and no controlled studies of it at all yet in musician’s focal dystonia. Nonetheless, I include some links below, to make it convenient for you to explore on your own, or keep tabs on the clinical trials’ status via the ClinicalTrials.gov website.
H owever, there is substantial published evidence regarding Xeomin®, the new formulation of botulinum toxin—one that does not contain significant amounts of complexing proteins and that therefore does not elicit undesired antibody production over the months that are required for effective dystonia treatment.
C ompared to the 10% to 40% or higher secondary failure rates due to immunogenicity experienced with BoTox® or Dysport® conventional formulations over periods of 2 years’ treatment or longer, the rate for Xeomin®is significantly lower—apparently less than 7% based on the past 2 years’ observational evidence that has accrued thus far in a U.S. datawarehouse that I use in my health informatics “day job”.
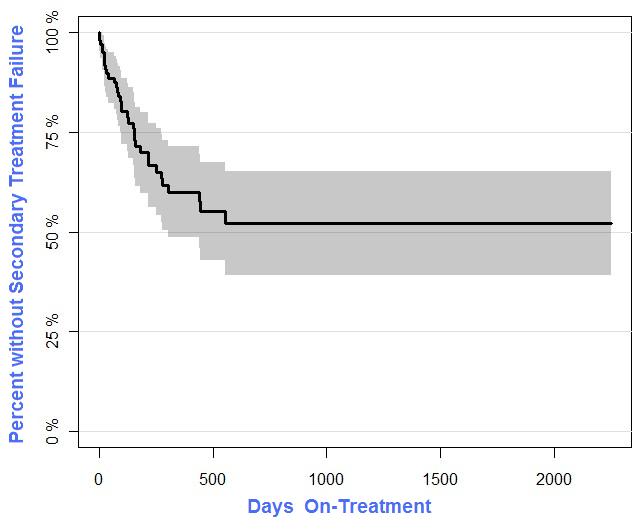
O ut of interest, I used the R statistical software package ‘prodlim’ to prepare a Kaplan-Meier regression for data on patients treated with conventional botulinum toxin formulations. Below is the result: a K-M plot of percentage without secondary failure, as a function of treatment duration in days. Basically, you have treatment failure if in the passing months your body produces antibodies that prevent the botulinum toxin from working and you have to stop the treatment because of the antibodies/hypersensitivity/non-efficacy prior to achieving successful resolution of your focal dystonia. You have “burned a bridge” insofar as the failed treatment has conditioned your body to make those antibodies against the botulinum toxin; in general, you can’t just go off-treatment for awhile and resume it: re-treatment with the drug will cause your immune system to make yet more antibodies and you’ll have treatment failure and perhaps worse immunotoxicity adverse events the next time around. Hence, the vigorous interest and the flurry of recent inquiries I’ve received here at CMT subsequent to FDA’s approval of Xeomin® 2010 and particularly since this month’s publication of Reiner Benecke’s journal article (link and pdf below).
- Reiner Benecke page at Klinik und Poliklinik für Neurologie, Universitätsmedizin Rostock
- Dystonia Medical Research Foundation website
- Previous CMT blogposts about focal dystonia
- Clinical trials on focal dystonia at ClincalTrials.gov
- Clinical trials on minocycline at ClinicalTrials.gov
- R system package 'prodlim' [Kaplan-Meier product-limit regression, etc.]
- Altenmüller E, Jabusch H. Focal hand dystonia in musicians: Phenomenology, etiology, and psychological trigger factors. J Hand Ther. 2009;22:144-54.
- Batla A, et al. Treatment of Focal Dystonia. Curr Treat Options Neurol. 2012 Mar 14. [Epub ahead of print]
- Barrett M, Bressman S. Genetics and pharmacological treatment of dystonia. Int Rev Neurobiol. 2011;98:525-49.
- Benecke R. Clinical relevance of botulinum toxin immunogenicity. BioDrugs. 2012 Apr 1;26:e1-9.
- Blum D, et al. Clinical potential of minocycline for neurodegenerative disorders. Neurobiol Dis. 2004;17:359-66.
- Bonelli R, et al. Neuroprotection in Huntington's disease: A 2-year study on minocycline. Int Clin Psychopharmacol. 2004;19:337-42.
- Byl A. Diagnosis and management of focal hand dystonia in a rheumatology practice. Curr Opin Rheumatol. 2012;24:222-31.
- Colosimo C, et al. Efficacy and safety of long-term botulinum toxin treatment in craniocervical dystonia: A systematic review. Neurotox Res. 2012 Feb 23. [Epub ahead of print]
- Dressler D. Clinical features of antibody-induced complete secondary failure of botulinum toxin therapy. Eur Neurol 2002;48:26-9.
- Fleisher L, Midgette A. My Nine Lives: A Memoir of Many Careers in Music. Doubleday, 2010.
- Garcia de Yebenes J. Did Robert Schumann have dystonia? Mov Disord. 1995;10:413-7.
- Jankovic J, et al. Comparison of efficacy and immunogenicity of original versus current botulinum toxin in cervical dystonia. Neurology. 2003;60:1186-8.
- Kim H, Suh Y. Minocycline and neurodegenerative diseases. Behav Brain Res. 2009;196:168-79.
- Lauterbach E, et al. Psychopharmacological neuroprotection in neurodegenerative disease. J Neuropsychiatry Clin Neurosci. 2010;22:8-18.
- Lederman R. Robert Schumann's focal dystonia. Semin Neurol. 1999;19(Suppl 1):17-24.
- Jimenez-Shahed J. A new treatment for focal dystonias: incobotulinumtoxinA (Xeomin®). Neuropsychiatr Dis Treat. 2012;8:13-25.
- Ochsner F. The musician’s cramp: About the illness of Robert Schumann. Rev Med Suisse. 2012;8:66-9.=
- Sandyk R, Kay S. Melatonin secretion and the pathophysiology of Meige’s disease (idiopathic orofacial dystonia). Funct Neurol. 1990;5:165-70.
- Truong D. Botulinum toxins in the treatment of primary focal dystonias. J Neurol Sci. 2012 Feb 13. [Epub ahead of print]
- Wang X. The antiapoptotic activity of melatonin in neurodegenerative diseases. CNS Neurosci Ther. 2009;15:345-57.
- Yong V, et al. The promise of minocycline in neurology. Lancet Neurol. 2004;3:744-51.
- Xeomin at RxList.com
- Xeomin® prescribing information (Merz Pharmaceuticals LLC)
- Dysport® prescribing information (Ipsen Ltd)
- BoTox® prescribing information (Allergan Inc)
No comments:
Post a Comment